RBQM Risk Assessment Risk Management Central Monitoring Quality Tolerance Limits Site Management
Made Simple
TRI's platform makes RBQM simple for all clinical trials. Think less complexity, more clarity. Think less spreadsheets, more innovation. Think OPRA.

- 90-day free trial
- No credit card required




ABOUT OPRA
OPRA, Pioneering RBQM for Clinical Trial Excellence
Meet OPRA, TRI's Risk-Based Quality Management (RBQM) platform. The most flexible, yet easy to use RBQM platform on the market. Featuring three core modules: OPRA-RAM, OPRA-CM, and OPRA-SM—used individually or in combination to meet the needs of each trial. OPRA revolutionizes RBQM with a comprehensive, systematic, and GCP-aligned approach. Whether you are just starting out or seeking advanced capabilities, OPRA will simplify RBQM for your clinical trials, improving trial integrity, efficiency and patient safety. Seamlessly integrating risk management throughout the trial lifecycle, and powered by advanced statistics and analytics, OPRA facilitates collaboration, decision-making, and the overall operationalization of RBQM.
-
Deployed on over 400 clinical trials
-
Secure, Validated, 21 CFR part 11 compliant
-
Designed for Sponsors and CROs running all trial phases

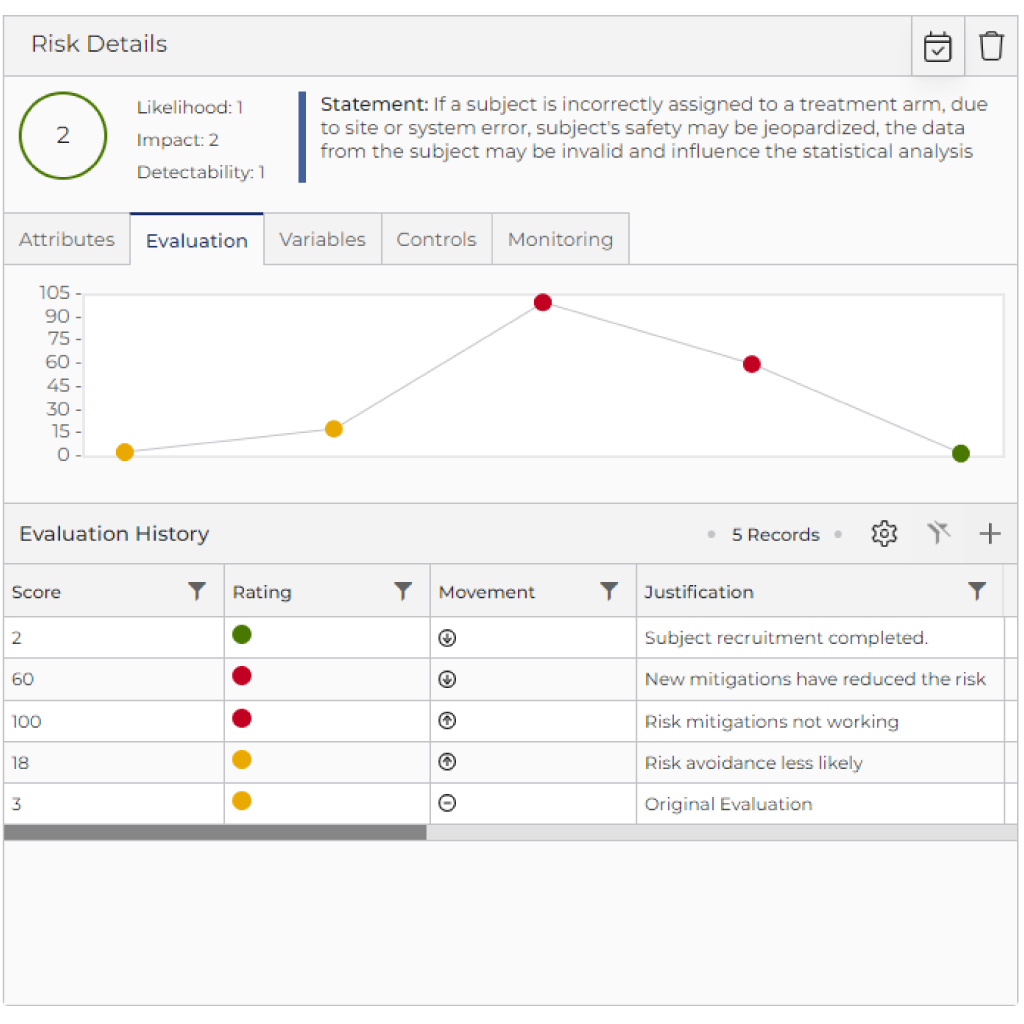
RISK ASSESSMENT AND MANAGEMENT
Empowering trials with OPRA, the future of RBQM
OPRA-RAM is the ultimate clinical trial risk management tool, enabling quality to be designed in and managed throughout the lifecycle of the trial. Drawing inspiration from the foundational principles of ICH E6 and E8, OPRA-RAM is meticulously designed to meet the requirements of GCP whilst providing an operational user experience which delivers clear, actionable insights. This is not merely about mitigating risks; it's about pioneering a transformative journey towards integrated quality, ensuring that every facet of your trial is aligned with the pinnacle of clinical excellence.
-
End to end quality and risk management
-
Strategic monitoring planning
-
Collaboration, activity management and reporting
CENTRALIZED MONITORING
Where innovation simplifies RBQM complexity
With OPRA-CM, the playbook for clinical trial monitoring is completely reimagined. Designed by Clinical Operations experts OPRA-CM provides a platform which is tailored to the specific needs of each clinical trial. This ensures laser-focus on what truly counts: proactively identifying and mitigating risks, ensuring the integrity of your study, and eliminating the burdens often associated with conventional monitoring methods. Tailored to meet the demands of modern trials, regardless of their scale, phase, location, OPRA-CM provides a smarter approach to quality management, where every decision is based on evidence.
-
Tailored for each individual trial
-
Easy to interpret insights
-
Collaboration, activity management and reporting
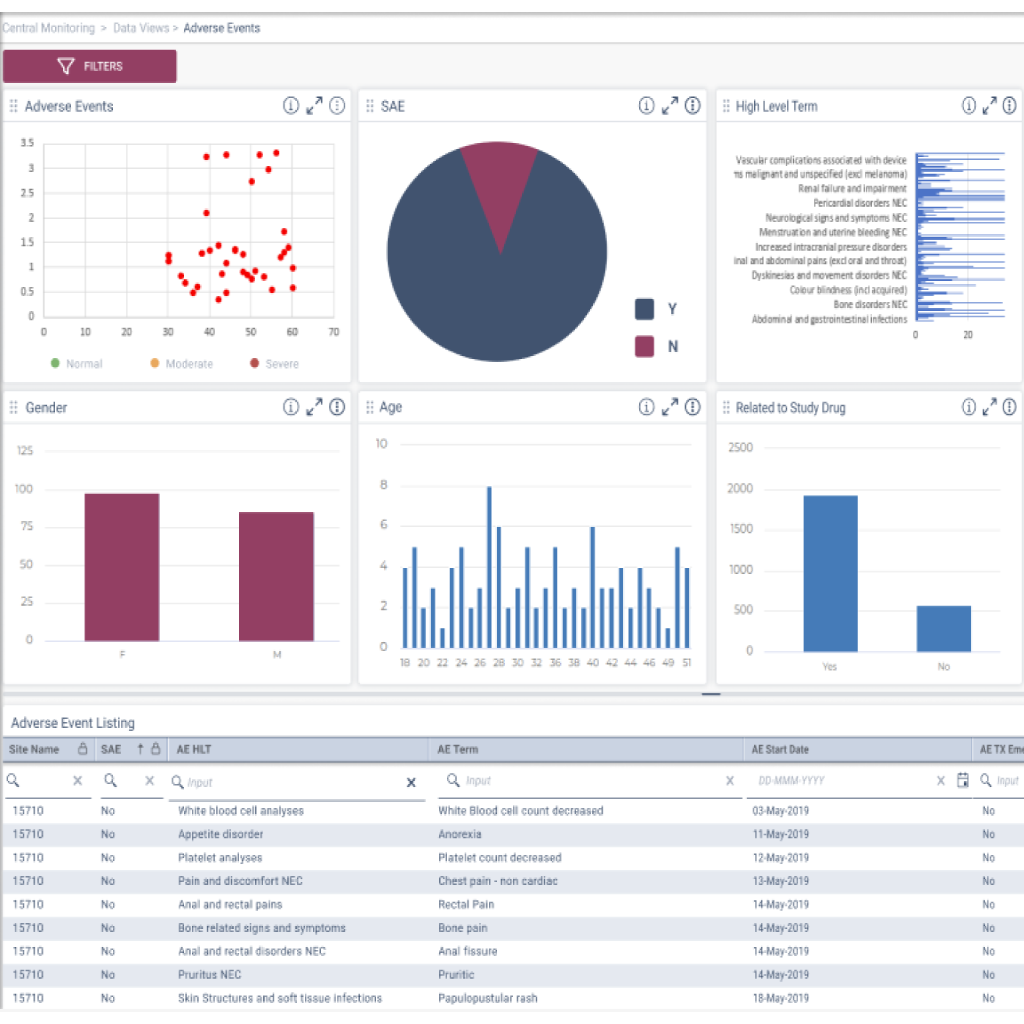
SUBJECT MONITORING
Subject Monitoring Precision in RBQM
In early phase trials, the ability to oversee the safety and progress of each patient is paramount. OPRA-SM is designed to idenitify and manage patient risk, where traditional site-based Central Monitoring approaches don't work. Medical monitoring of patient data can be inefficient and error-prone, but those problems go away with OPRA-SM's unique views on data changes and patient journeys.
-
Enhanced patient safety
-
Improved data integrity
-
Greater operational efficiency
Compare OPRA
to traditional monitoring solutions
Traditional Monitoring
OPRA-CM
Proactive
Proactively identifies and addresses risks throughout the trial, preventing issues before they occur.
Comprehensive
Takes a holistic approach, considering risks across the entire trial, from protocol design to data analysis.
Data-Driven
Relies on data and analytics to make informed decisions about risk mitigation.
Flexible
Adapts to changes and unforeseen circumstances, maintaining quality in trials.
Adaptable
Allows for tailored risk assessments and mitigation strategies based on trial-specific factors.
Transparent
Promotes transparency by documenting risk assessments, mitigation plans, and decision-making processes.
Frequently asked questions
We've got the answer
Explore our FAQs for answers and expert advice on RBQM.
TESTIMONIALS
RBQM Success Stories
Hear directly from our partners about how we've helped them.


What truly set TriTrials apart from other vendors was their unparalleled commitment to a quality deliverable helping us effectively apply a novel approach to our operations. They achieved a deep understanding of our objectives by partnering with us on a rigorous change management process which culminated in a shared vision for the integration of their risk assessment and central monitoring solution.

The Worldwide Clinical Trials team is pleased with our continued partnership with TRI and we are excited about the milestone of having 150 clinical trials in TRI's Risk-Based Quality Management platform. This is a key benefit for our customers as it improves data quality, trial efficiency, and patient safety.
RBQM NEWS
Clinical trial quality news and views
Subscribe to get the latest RBQM insights sent to your mailbox.
Dose Optimization in Oncology: Advancing Clinical Trial Quality through Risk-Based Approaches
The complexities of oncology drug development have long posed significant challenges, particularly in determining the optimal dose that balances therapeutic efficacy with patient safety. The FDA’s recent guidance on dose…
TRI Joins Veeva’s Product Partner Program
Partnership will drive quality and efficiency in clinical trials through enhanced risk-based quality management integration. [Cambridge, UK – 17.10.2024] – TRI, a leading Risk-Based Quality Management (RBQM) software provider for…
Risk-Based Clinical Data Management Takes Center Stage at SCDM 2024
The Society for Clinical Data Management (SCDM) annual conference has always been a cornerstone for clinical data professionals, but this year’s event, held in Boston from September 30th to October…









